Abstract
Introduction
Mantle-cell lymphoma (MCL) is a B-cell non-Hodgkin Lymphoma (NHL) characterized by heterogenous behavior, ranging from indolent phenotype to highly aggressive and drug resistant one with dismal prognosis. Drug resistance may be generated by Tumor Microenvironment (TME), owing that Tumor-Associated Macrophages (TAM) are pathologically functional in providing survival signals to MCL cells (Pham, Front Oncol. 2018).
Recently, "Don't Eat Me" signal (DEMs) blockade with anti-CD47 monoclonal Antibody (moAb) showed promising activity in pretreated NHL, through increase of phagocytosis by TAM (Advani, NEJM. 2019).
CD24 was also demonstrated to be involved in DEMs and, in a preclinical model of solid cancer, blocking the CD24/Siglec-10 interaction provided an improvement of M2-like TAM-mediated phagocytosis in vitro and an increase of survival in vivo (Barkal, Nature. 2019).
CD24 can be expressed in some phases of B-cell differentiation and MCL derives from a B-cell precursor with upregulated CD24. To date, there are no functional studies showing an improvement of phagocytosis through CD24/Siglec-10 pathway inhibition in hematologic malignancies and MCL.
Here, we present our in vitro results of CD24/Siglec-10 DEMs blockade in MCL subset.
Methods
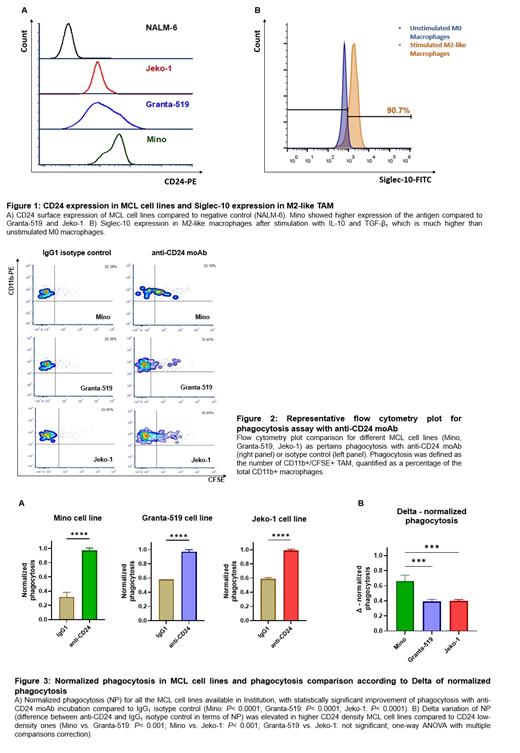
A panel of MCL cell lines (Jeko-1, Granta-519, Mino) has been analyzed for CD24 surface expression by flow cytometry (FC) (clone SN3). Consequently, we performed co-culture experiments with MCL cell lines and macrophages from healthy donors. Briefly, Peripheral Blood Mononucleated Cells (PBMC) were collected from healthy volunteers through density gradient centrifugation technique. CD14+ monocytes were isolated through CD14 Microbeads isolation kit and cultured in plates with 50 ng/ml human GM-CSF for 7-9 days. In order to create M2-like Siglec-10+ TAM, 50 ng/ml human IL-10 and 50 ng/ml human TGF-β 1 were added on days 3-4 of differentiation until use on days 7-9. Siglec-10 expression on TAM was checked by FC (clone 5G6).
M2-like macrophages were then collected and co-cultured with CFSE-labelled MCL target cells for 1-2 hours in a serum-free medium. Anti-CD24 moAb (clone SN3) or the appropriate IgG 1 isotype control were added at a concentration of 10 μg/ml. Phagocytosis was then stopped on ice and CD11b-PE staining (anti-CD11b moAb, clone REA713) was performed to identify human macrophages by FC.
Phagocytosis was measured as the number of CD11b+/CFSE+ macrophages, quantified as a percentage of the total CD11b+ macrophages. Each phagocytosis reaction was performed in technical triplicate and phagocytosis was normalized to the highest technical replicate per donor in order to consider raw phagocytic level among donor-derived macrophages.
Results
MCL cell lines express surface CD24 by FC, with higher levels in Mino cell line (Figure 1A). Differentiated M2-like macrophages showed an upregulation of Siglec-10 expression after immunosuppressive stimuli, which is fundamental owing that Siglec-10 is the ligand of CD24 (Figure 1B).
As pertains to the phagocytic assay, we documented an improvement of phagocytosis when M2-like macrophages and MCL cell lines were co-cultured together with anti-CD24 moAb (Figure 2 and Figure 3A). Furthermore, it is worth mentioning that phagocytosis seemed to be much higher in MCL cell lines with higher surface levels of CD24 (e.g., Mino), presenting increased number of CD11b+/CFSE+ M2-like TAM by FC (Figure 3B).
Conclusions
MCL was found to be sensitive to CD24/Siglec-10 DEMs blockade when co-cultured with M2-like macrophages in vitro. We can argue that most of the observed increase of phagocytosis after the addition of anti-CD24 moAb may be secondary to loss of CD24 signalling rather than Fc-mediated opsonization, as already documented in previous analysis about solid cancer (Barkal, Nature. 2019). We can therefore hypothesize that the blockade of this DEMs pathway can improve phagocytosis in a non-opsonization manner in NHL as well.
Furthermore, CD24 surface density seemed to be positively correlated to the intensity of phagocytic activity, suggesting that MCL subtypes expressing higher CD24 levels are much more dependent on this DEMs pathway than others with low CD24 density.
Overall, CD24 turned out to be a potential immunotherapeutic target in MCL, aiming at improving innate immune system through DEMs blockade. In vivo studies are needed to confirm the activity we documented in vitro in this NHL subset.
Gambacorti-Passerini: Bristol-Myers Squibb: Consultancy; Pfizer: Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal